B. Braun 1.2 Micron Filtered Extension Set with CARESITE Injection Site
Buy more to unlock extra rewards!
PayPal Pay Later
Free Shipping
Description
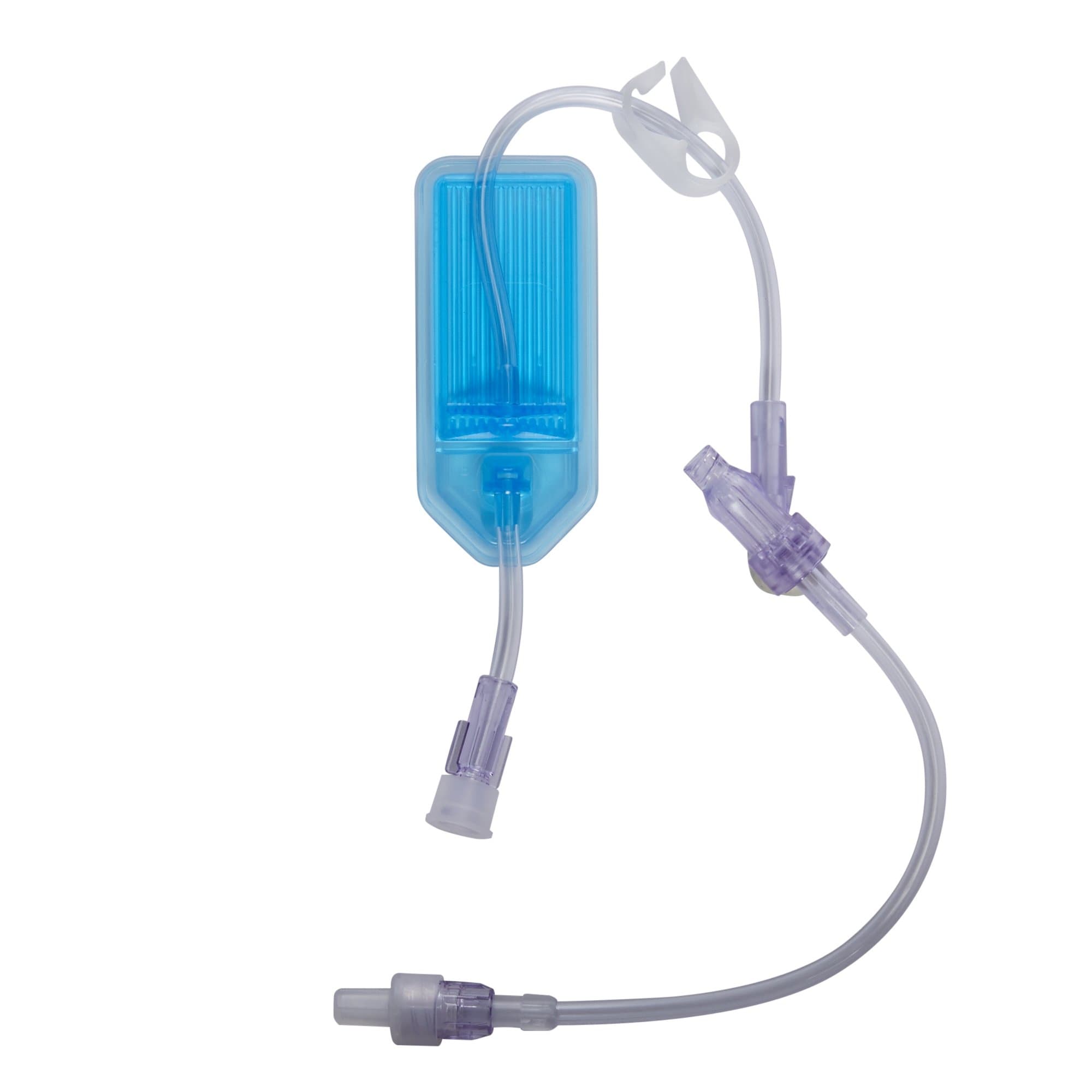
The B. Braun 1.2 Micron Filtered Extension Set with CARESITE Injection Site is a critical medical device designed to deliver safe, sterile, and reliable intravenous fluid and medication administration in healthcare settings. This extension set combines advanced filtration technology with needle-free access, making it an ideal choice for clinical professionals who prioritize patient safety and ease of use.
Product Overview and Purpose
This filtered IV extension set is engineered for use in hospitals, clinics, ambulatory care centers, and other medical facilities where reliable intravenous access is essential. The 1.2 micron filter works to eliminate air bubbles and protect against particulate contamination, ensuring that medications and fluids reach patients safely. The CARESITE needle-free injection site provides convenient, secure access for multiple medication administrations without the need for traditional needles, reducing the risk of needlestick injuries and improving infection control.
Key Features and Benefits
- Advanced 1.2 Micron Filtration: Provides effective air elimination and particulate protection, safeguarding fluid integrity during administration.
- CARESITE Needle-Free Injection Site: Located 6 inches above the distal end, this port allows for multiple safe medication accesses without traditional needle puncture.
- Optimal Tubing Dimensions: The 16-inch tubing length and 4.5 mL priming volume offer a balance between patient flexibility and minimal fluid waste.
- SPIN-LOCK Connector: Ensures secure, leak-free connections to reduce the risk of line displacement or fluid extravasation.
- Flow Control: On/off clamp allows for easy regulation of fluid flow rates according to clinical requirements.
- Patient Safety Materials: DEHP-free and latex-free composition reduces allergic reactions and chemical exposure risks for sensitive patients.
Material Composition and Safety
The tubing is constructed from latex-free, DEHP-free PVC that meets rigorous safety standards. While the product contains BPA and PVC as structural components, the absence of DEHP (a plasticizer) and natural rubber latex makes it suitable for patients with known sensitivities. This formulation supports clinical protocols in facilities that prioritize the avoidance of potentially harmful chemical additives.
Sterility and Shelf Life
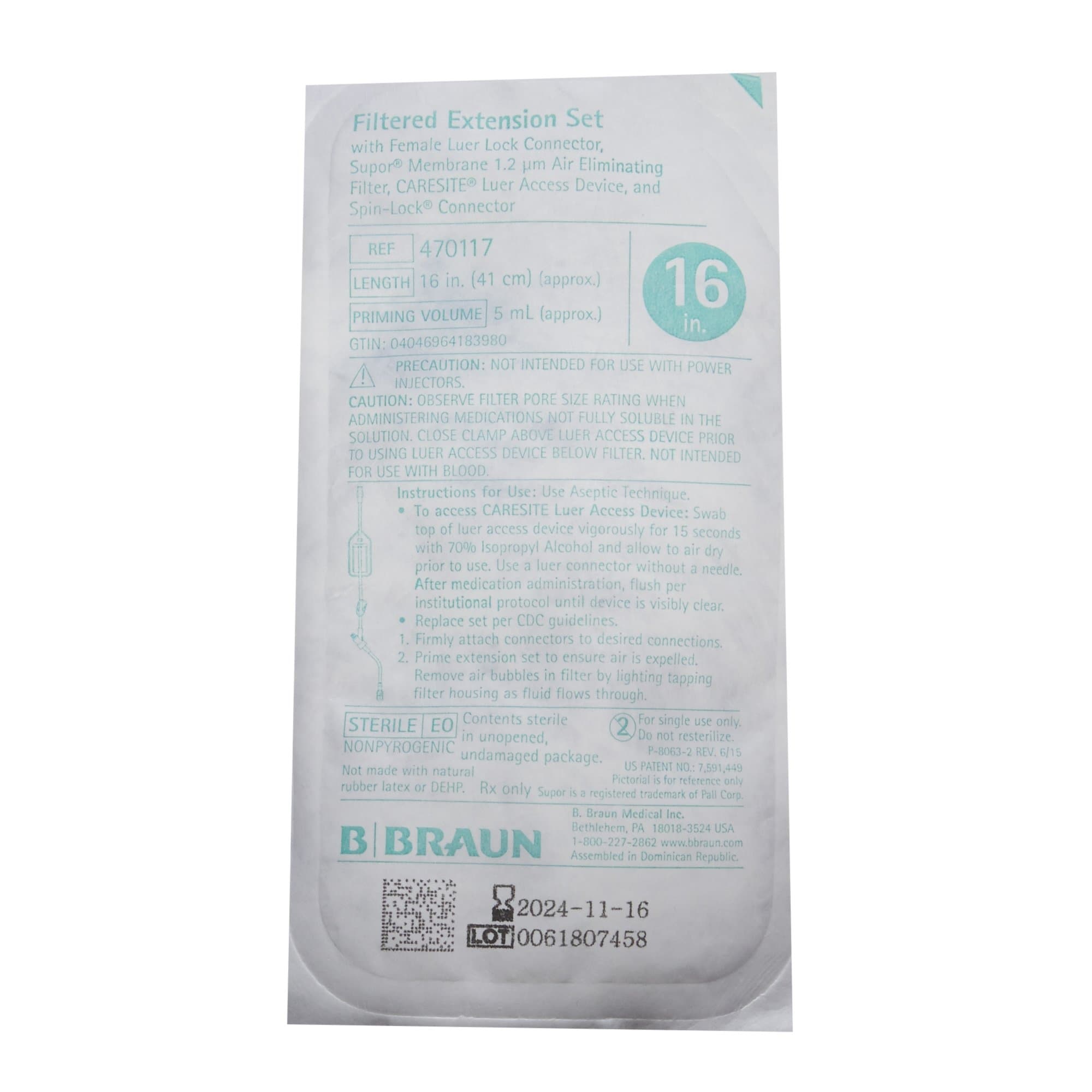
Each set is sterilized using ethylene oxide (EO) and comes in sterile, single-use packaging. The 36-month shelf life from the date of manufacture provides an adequate timeframe for inventory management in busy healthcare facilities. Always verify expiration dates before clinical use to ensure product integrity and maintain patient safety standards.
Who This Product Is For
This extension set is suitable for adult patients requiring intravenous fluid or medication administration in clinical settings. It is particularly beneficial for patients requiring multiple medication accesses, those sensitive to latex or DEHP exposure, and facilities implementing needle-free protocols to reduce occupational exposures among healthcare workers.
Storage and Handling
Store in original sterile packaging at room temperature in a cool, dry location. Protect from direct sunlight and extreme temperatures to maintain sterility and product efficacy. Do not open or use if the packaging is visibly compromised. Follow your facility's established protocols for inventory rotation and expiration date management.
Specifications
| Brand | FilterFlow |
| Manufacturer | B. Braun |
| Application | IV Extension Set |
| Back Check Valve | Without Back Check Valve |
| Connection Type | Spin-Lock Connector |
| DEHP Indicator | DEHP-Free |
| Filter | 1.2 Micron Filter |
| Flow Control Type | Pinch Clamp |
| HCPCS | S1015 |
| Injection Port Type | CARESITE Valve Injection |
| NDC Number | 08021470117 |
| Number of Ports | 1 Port |
| Priming Volume | 5 mL Priming Volume |
| Sterility | Sterile |
| Tubing Length | 16 Inch Tubing |
| UNSPSC Code | 42221616 |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
FAQs
Q: What is the purpose of the 1.2 micron filter on this IV extension set?
A: The 1.2 micron filter helps eliminate air bubbles and provides protection against particulate contamination during IV fluid administration, ensuring safer delivery of medications and fluids to patients.
Q: Is this extension set suitable for use with all IV pump types?
A: This extension set is designed for gravity-based IV administration. Compatibility with specific infusion pump models may vary. Always verify pump compatibility with the manufacturer or your clinical team before use.
Q: Can the CARESITE injection site be used multiple times for medication administration?
A: The CARESITE needle-free injection site allows for multiple accesses during the same infusion session. However, follow your facility's protocols for access frequency and aseptic technique to prevent contamination.
Q: Why is this set DEHP-free and latex-free?
A: DEHP-free and latex-free materials reduce the risk of allergic reactions and harmful chemical exposure, making the product safer for patients, especially those with sensitivities or allergies to these substances.
Q: What is the shelf life of this sterile extension set?
A: The total shelf life is 36 months from the date of manufacture. Always check the expiration date on the packaging before use to ensure product integrity and sterility.
Q: How should this extension set be stored?
A: Store in a cool, dry place at room temperature. Protect from direct sunlight and extreme temperatures. Keep in original sterile packaging until ready for use to maintain sterility.
Description
The B. Braun 1.2 Micron Filtered Extension Set with CARESITE Injection Site is a critical medical device designed to deliver safe, sterile, and reliable intravenous fluid and medication administration in healthcare settings. This extension set combines advanced filtration technology with needle-free access, making it an ideal choice for clinical professionals who prioritize patient safety and ease of use.
Product Overview and Purpose
This filtered IV extension set is engineered for use in hospitals, clinics, ambulatory care centers, and other medical facilities where reliable intravenous access is essential. The 1.2 micron filter works to eliminate air bubbles and protect against particulate contamination, ensuring that medications and fluids reach patients safely. The CARESITE needle-free injection site provides convenient, secure access for multiple medication administrations without the need for traditional needles, reducing the risk of needlestick injuries and improving infection control.
Key Features and Benefits
- Advanced 1.2 Micron Filtration: Provides effective air elimination and particulate protection, safeguarding fluid integrity during administration.
- CARESITE Needle-Free Injection Site: Located 6 inches above the distal end, this port allows for multiple safe medication accesses without traditional needle puncture.
- Optimal Tubing Dimensions: The 16-inch tubing length and 4.5 mL priming volume offer a balance between patient flexibility and minimal fluid waste.
- SPIN-LOCK Connector: Ensures secure, leak-free connections to reduce the risk of line displacement or fluid extravasation.
- Flow Control: On/off clamp allows for easy regulation of fluid flow rates according to clinical requirements.
- Patient Safety Materials: DEHP-free and latex-free composition reduces allergic reactions and chemical exposure risks for sensitive patients.
Material Composition and Safety
The tubing is constructed from latex-free, DEHP-free PVC that meets rigorous safety standards. While the product contains BPA and PVC as structural components, the absence of DEHP (a plasticizer) and natural rubber latex makes it suitable for patients with known sensitivities. This formulation supports clinical protocols in facilities that prioritize the avoidance of potentially harmful chemical additives.
Sterility and Shelf Life
Each set is sterilized using ethylene oxide (EO) and comes in sterile, single-use packaging. The 36-month shelf life from the date of manufacture provides an adequate timeframe for inventory management in busy healthcare facilities. Always verify expiration dates before clinical use to ensure product integrity and maintain patient safety standards.
Who This Product Is For
This extension set is suitable for adult patients requiring intravenous fluid or medication administration in clinical settings. It is particularly beneficial for patients requiring multiple medication accesses, those sensitive to latex or DEHP exposure, and facilities implementing needle-free protocols to reduce occupational exposures among healthcare workers.
Storage and Handling
Store in original sterile packaging at room temperature in a cool, dry location. Protect from direct sunlight and extreme temperatures to maintain sterility and product efficacy. Do not open or use if the packaging is visibly compromised. Follow your facility's established protocols for inventory rotation and expiration date management.
Specifications
| Brand | FilterFlow |
| Manufacturer | B. Braun |
| Application | IV Extension Set |
| Back Check Valve | Without Back Check Valve |
| Connection Type | Spin-Lock Connector |
| DEHP Indicator | DEHP-Free |
| Filter | 1.2 Micron Filter |
| Flow Control Type | Pinch Clamp |
| HCPCS | S1015 |
| Injection Port Type | CARESITE Valve Injection |
| NDC Number | 08021470117 |
| Number of Ports | 1 Port |
| Priming Volume | 5 mL Priming Volume |
| Sterility | Sterile |
| Tubing Length | 16 Inch Tubing |
| UNSPSC Code | 42221616 |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
FAQs
Q: What is the purpose of the 1.2 micron filter on this IV extension set?
A: The 1.2 micron filter helps eliminate air bubbles and provides protection against particulate contamination during IV fluid administration, ensuring safer delivery of medications and fluids to patients.
Q: Is this extension set suitable for use with all IV pump types?
A: This extension set is designed for gravity-based IV administration. Compatibility with specific infusion pump models may vary. Always verify pump compatibility with the manufacturer or your clinical team before use.
Q: Can the CARESITE injection site be used multiple times for medication administration?
A: The CARESITE needle-free injection site allows for multiple accesses during the same infusion session. However, follow your facility's protocols for access frequency and aseptic technique to prevent contamination.
Q: Why is this set DEHP-free and latex-free?
A: DEHP-free and latex-free materials reduce the risk of allergic reactions and harmful chemical exposure, making the product safer for patients, especially those with sensitivities or allergies to these substances.
Q: What is the shelf life of this sterile extension set?
A: The total shelf life is 36 months from the date of manufacture. Always check the expiration date on the packaging before use to ensure product integrity and sterility.
Q: How should this extension set be stored?
A: Store in a cool, dry place at room temperature. Protect from direct sunlight and extreme temperatures. Keep in original sterile packaging until ready for use to maintain sterility.
