
**The images used on the website are for reference only and do not reflect the actual configuration of the product.
Image 1 of 3. Swipe to navigate. Tap to view fullscreen.
Biogel PI UltraTouch G Sterile Polyisoprene Surgical Gloves
SKU:MO42160-MI
MPN:42160
PayPal Pay Later
Pay low as $9.29/month with Pay Pal's pay later
Starts From
$202.81
$212.95Save $10.14Subscribe & Save
Save 5%$192.67$202.81
Secure transaction
Your payment information is processed securely.
Description
Biogel PI UltraTouch G Sterile Polyisoprene Surgical Gloves Latex-free, micro-roughened, and chemotherapy-tested for superior protection, grip, and all-day confidence in high-risk procedures. Experience unmatched precision and comfort in every surgery.
Features:
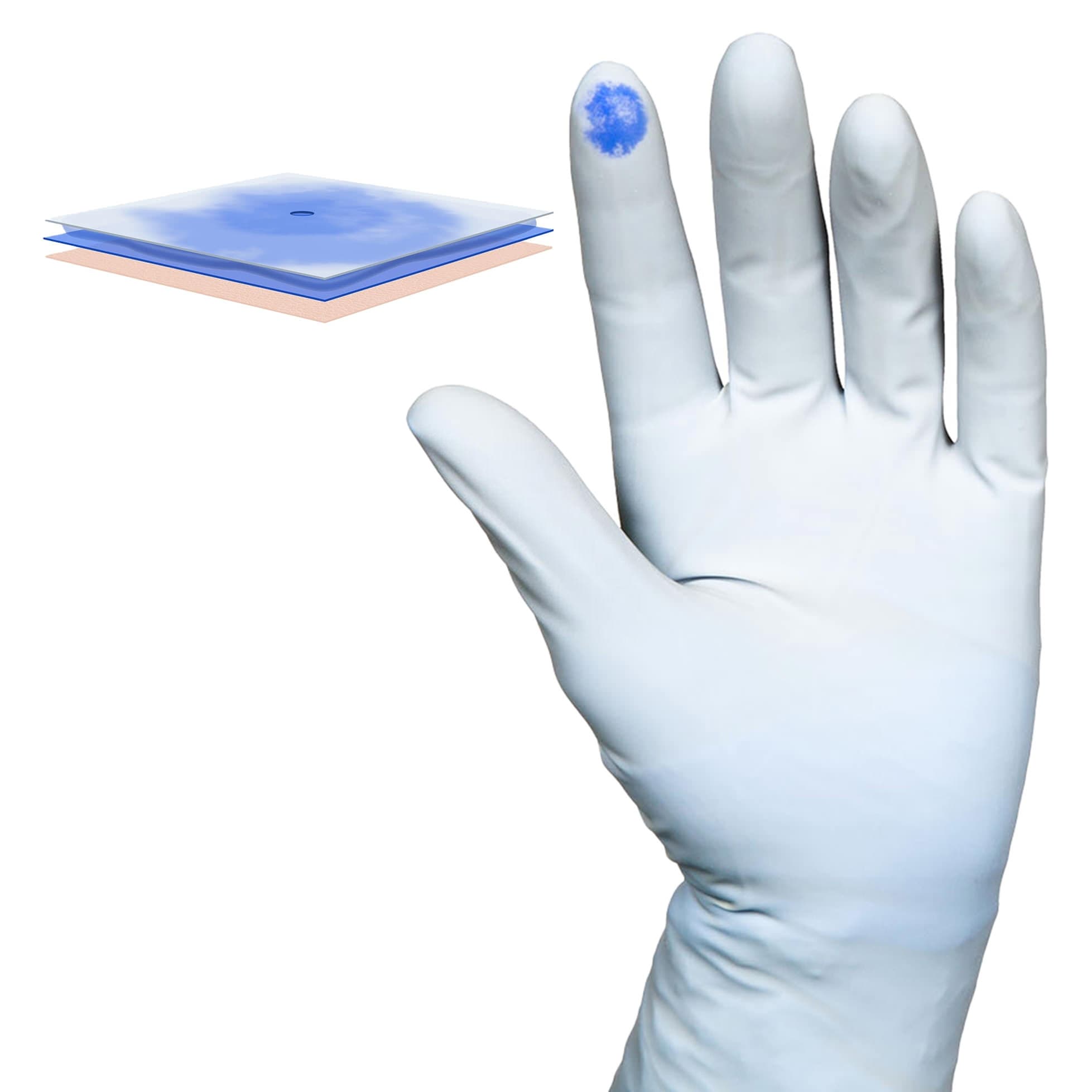
- Enhanced Sensitivity and Grip: Micro-roughened surface improves grip and tactile sensitivity, allowing precise handling of surgical instruments.
- Latex-Free Protection: Made from synthetic polyisoprene to reduce the risk of latex protein sensitization for both patients and clinicians.
- Biogel Coating: Allows easy donning even with damp hands and helps maintain skin moisture for comfort during long procedures.
- Ergonomic Design: Biomechanically engineered to reduce hand and thumb fatigue during prolonged surgeries.
- Chemotherapy Tested: Approved for use with chemotherapy agents, providing safe protection during oncology procedures.
- Intended Use: Recommended for orthopedic, microvascular, cardiothoracic, ENT, plastic, neurosurgical, gynecological, and general surgeries. Ideal where high barrier protection and tactile precision are needed.
- Packaging & Compliance: Sterile, powder-free meets ISO 9001, ISO 13485, ISO 14001, ASTM D3577, EN455 series, and CE Class IIa standards.
Specifications
| Manufacturer | Molnlycke |
| Manufacturer # | 42160 |
| Application | Surgical Glove |
| Color | Straw |
| Color Family | Neutral |
| Cuff Style | Beaded Cuff |
| Fingertip Thickness Range | Standard |
| Fingertip Thickness | 0.27 mm (10.6 mil) |
| Glove Exterior | Micro-Textured |
| Glove Interior | With Polymer Coating |
| Glove Length | Standard Cuff Length |
| Hand Compatibility | Hand Specific |
| Length | 11.2 Inch |
| Material | Polyisoprene |
| Protection Level | Chemo Tested |
| UNSPSC Code | 42132205 |
| Sterility | Sterile |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Description
Biogel PI UltraTouch G Sterile Polyisoprene Surgical Gloves Latex-free, micro-roughened, and chemotherapy-tested for superior protection, grip, and all-day confidence in high-risk procedures. Experience unmatched precision and comfort in every surgery.
Features:
- Enhanced Sensitivity and Grip: Micro-roughened surface improves grip and tactile sensitivity, allowing precise handling of surgical instruments.
- Latex-Free Protection: Made from synthetic polyisoprene to reduce the risk of latex protein sensitization for both patients and clinicians.
- Biogel Coating: Allows easy donning even with damp hands and helps maintain skin moisture for comfort during long procedures.
- Ergonomic Design: Biomechanically engineered to reduce hand and thumb fatigue during prolonged surgeries.
- Chemotherapy Tested: Approved for use with chemotherapy agents, providing safe protection during oncology procedures.
- Intended Use: Recommended for orthopedic, microvascular, cardiothoracic, ENT, plastic, neurosurgical, gynecological, and general surgeries. Ideal where high barrier protection and tactile precision are needed.
- Packaging & Compliance: Sterile, powder-free meets ISO 9001, ISO 13485, ISO 14001, ASTM D3577, EN455 series, and CE Class IIa standards.
Specifications
| Manufacturer | Molnlycke |
| Manufacturer # | 42160 |
| Application | Surgical Glove |
| Color | Straw |
| Color Family | Neutral |
| Cuff Style | Beaded Cuff |
| Fingertip Thickness Range | Standard |
| Fingertip Thickness | 0.27 mm (10.6 mil) |
| Glove Exterior | Micro-Textured |
| Glove Interior | With Polymer Coating |
| Glove Length | Standard Cuff Length |
| Hand Compatibility | Hand Specific |
| Length | 11.2 Inch |
| Material | Polyisoprene |
| Protection Level | Chemo Tested |
| UNSPSC Code | 42132205 |
| Sterility | Sterile |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Starts From
$202.81
$212.95Save $10.14Subscribe & Save
Save 5%$192.67$202.81
Secure transaction
Your payment information is processed securely.
