
NeoMed PU Nasogastric Enteral Feeding Tube with ENFit Connector
Starts From
Description
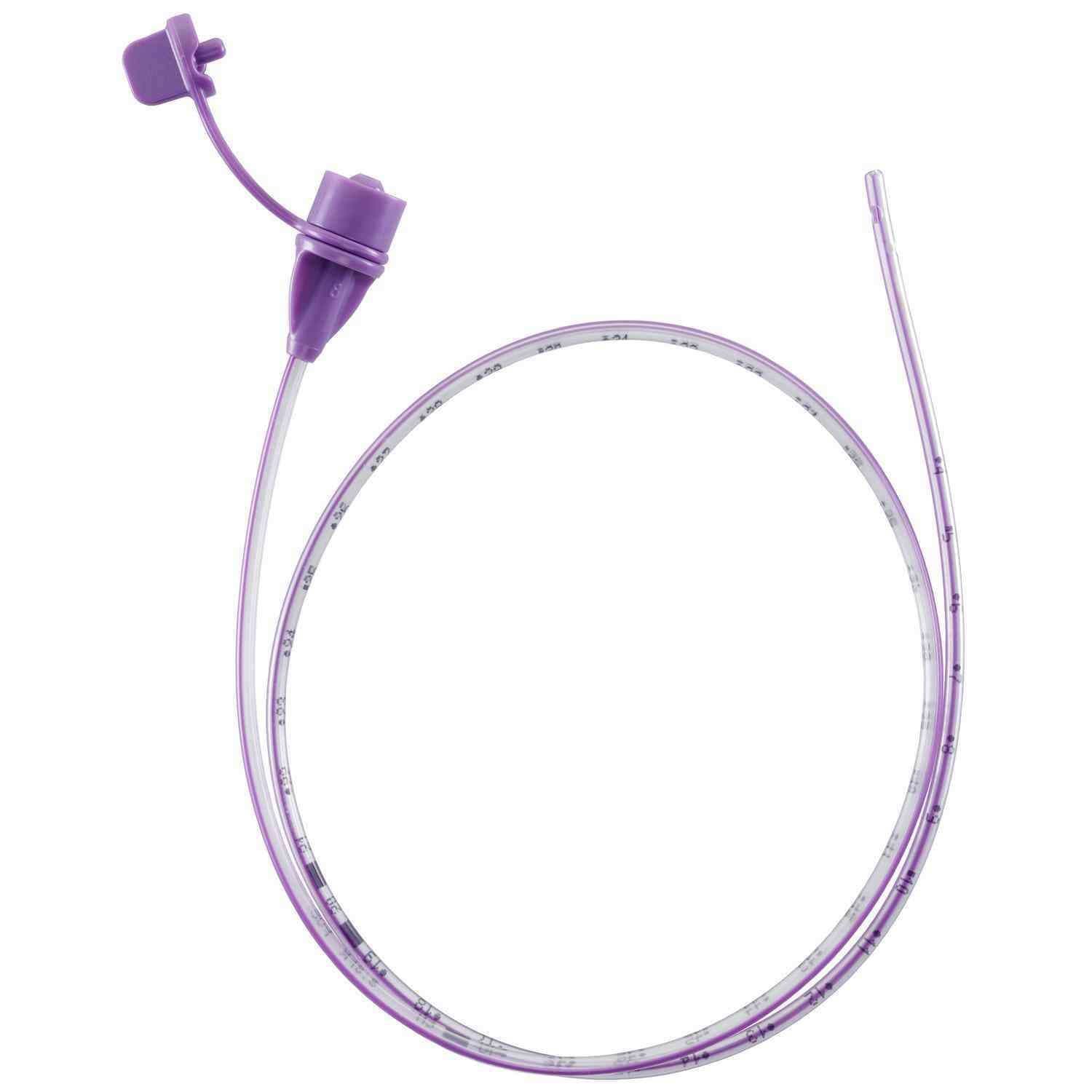
NeoMed Polyurethane Enteral Feeding Tube is engineered to support gentle nasogastric and/or orogastric nutrition delivery while helping minimize tissue irritation commonly associated with oversized side ports or unfinished distal tips. The distal end is designed to remain smooth, open, and well-formed, while exposed surfaces are structured for accessibility and effective cleaning. Thoughtful hub and flow-path design helps limit pooling that may contribute to bacterial transfer, and the polyurethane construction delivers a balance of flexibility and durability for neonatal and pediatric clinical use.
Features
- BPA-free materials selected for patient safety.
- Tethered cap configuration to help protect the lumen when not in use.
- Radiopaque stripe to assist with radiographic visualization.
- ENFit connector to support standardized enteral connections.
- An anti-IV connector to help reduce the risk of misconnections.
- DEHP-free construction.
- Centimeter markings to at least 32 cm for insertion guidance.
- Bold centimeter indicators to help confirm placement.
- Unique tethered plug closure designed to limit exposure to ambient air.
- Soft medical-grade polymers developed to support developmental care and clinical performance.
- Wave-pattern closure plug that allows airflow through the hub to help reduce bacterial formation.
- Bottomless hub design that limits the accumulation of HBM or formula.
- Cost-effective configuration validated for indwell time up to thirty days to help reduce repeat placements.
- Open the distal tip without sharp edges or hidden cavities to help limit residual colonization.
- Three offset side ports are precision drilled to deliver smooth edges compared to large cut openings.
Intended Users: This device is designed for use by trained healthcare professionals.
Patient Target Group: This device is intended for patients of all age groups requiring nasogastric or orogastric enteral feeding.
Indications for Use: This product is indicated for neonatal and pediatric patients to provide nutrition via nasal or oral gastric placement and is not intended for use beyond 30 days.
Contraindications: This device is designed to connect only with ENFit-compatible connectors. It is not intended for use longer than 30 days or for patients with congenital gastrointestinal anomalies above the stomach requiring surgical intervention.
Device Description / Intended Purpose: The NEOMED Enteral Feeding Tube is intended for patients who require intermittent or continuous enteral nutrition via the nasogastric or orogastric pathway. Material options include polyurethane. Refer to product packaging for product-specific information.
Residual Risks and Side Effects: While outcomes vary depending on the clinical procedure, mitigations have been implemented to help minimize the following potential risks associated with NEOMED usage.
Nasogastric Feeding Tubes and associated NEOMED Extension Sets: abnormal reproductive development, allergic or systemic toxic reaction, over or under delivery of medication or nutrition, skin or tissue irritation involving the trachea, vocal cords, or gastrointestinal tract, infection, fever, sepsis, nausea, vomiting, bloating, diarrhea, delayed or prolonged therapy, pneumothorax, choking, aspiration, peritonitis, gastroenteritis, laceration, pain, and potential long-term carcinogenic or mutagenic effects.
MR Information
MR Safe
The NEOMED Enteral Feeding Tube contains only nonconducting, nonmetallic, and nonmagnetic components and presents no known hazards in MR imaging environments.
Clinical Benefits: Nasogastric and/or orogastric feeding tubes facilitate delivery of nutrition, fluids, and medications to the stomach through the nose or mouth.
Device Lifetime: Device performance has been validated for use up to 30 days.
Recommended Placement Procedure
Insertion Measurement: measure from the tip of the nose to the earlobe and then from the earlobe to midway between the xyphoid process and the umbilicus. Centimeter markings assist with placement.
A strip of sterile tape may be placed at the intended insertion depth for reference.
Insert the tube slowly into the mouth or nose, allowing the infant to swallow as advancement continues.
Advance to the predetermined length.
Precautions: Avoid unintended tracheal placement or insufficient insertion that leaves the proximal side port outside the stomach. Secure the tube before checking placement.
Verify Feeding Tube Placement: Confirm and document positioning per facility protocol, such as chest radiograph or aspiration with pH testing. Reposition or remove if uncertain.
Recommended Maintenance
Inspect tube position before each administration using catheter markings.
Verify placement per hospital protocol.
Flush with sterile water before and after administration.
Seal with tethered closure when not in use.
Recommended Feeding Tube Removal
Flush with 0.5cc–1cc sterile water.
Plug or clamp during withdrawal to help minimize aspiration risk.
Withdraw gently and discard according to institutional or regulatory requirements.
Document removal.
Disposal: Follow local, state, and national regulations for disposal of potentially biohazardous components and accessories.
Specifications
| Product Code (SKU) | Diameter (Fr) | Inner Diameter (mm) | Length (cm) | Primary Product Color |
| FTL5.0P-NC | 5 | 0.9 | 90 | Orange |
| FTL6.5P-NC | 6.5 | 1.25 | 90 | Orange |
| FTL8.0P-NC | 8 | 1.6 | 90 | Orange |
| FTM10.0P-NC | 10 | 2.45 | 60 | Orange |
| FTM12.0P-NC | 12 | 2.65 | 60 | Orange |
| FTM5.0P-NC | 5 | 0.9 | 60 | Orange |
| FTM6.5P-NC | 6.5 | 1.25 | 60 | Orange |
| FTM8.0P-NC | 8 | 1.6 | 60 | Orange |
| FTS4.0P-NC | 4 | 0.6 | 40 | Orange |
| FTS5.0P-NC | 5 | 0.9 | 40 | Orange |
| FTS6.5P-NC | 6.5 | 1.25 | 40 | Orange |
| FTS8.0P-NC | 8 | 1.6 | 40 | Orange |
| FTXL5.0P-NC | 5 | 0.9 | 105 | Orange |
| FTXL8.0P-NC | 8 | 1.6 | 105 | Orange |
| PFTL5.0P-NC | 5 | 0.9 | 90 | Purple |
| PFTL6.5P-NC | 6.5 | 1.25 | 90 | Purple |
| PFTL8.0P-NC | 8 | 1.6 | 90 | Purple |
| PFTM10.0P-NC | 10 | 2.45 | 60 | Purple |
| PFTM12.0P-NC | 12 | 2.65 | 60 | Purple |
| PFTM5.0P-NC | 5 | 0.9 | 60 | Purple |
| PFTM6.5P-NC | 6.5 | 1.25 | 60 | Purple |
| PFTM8.0P-NC | 8 | 1.6 | 60 | Purple |
| PFTS4.0P-NC | 4 | 0.6 | 40 | Purple |
| PFTS5.0P-NC | 5 | 0.9 | 40 | Purple |
| PFTS6.5P-NC | 6.5 | 1.25 | 40 | Purple |
| PFTS8.0P-NC | 8 | 1.6 | 40 | Purple |
| PFTXL5.0P-NC | 5 | 0.9 | 105 | Purple |
| PFTXL8.0P-NC | 8 | 1.6 | 105 | Purple |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Resources
Description
NeoMed Polyurethane Enteral Feeding Tube is engineered to support gentle nasogastric and/or orogastric nutrition delivery while helping minimize tissue irritation commonly associated with oversized side ports or unfinished distal tips. The distal end is designed to remain smooth, open, and well-formed, while exposed surfaces are structured for accessibility and effective cleaning. Thoughtful hub and flow-path design helps limit pooling that may contribute to bacterial transfer, and the polyurethane construction delivers a balance of flexibility and durability for neonatal and pediatric clinical use.
Features
- BPA-free materials selected for patient safety.
- Tethered cap configuration to help protect the lumen when not in use.
- Radiopaque stripe to assist with radiographic visualization.
- ENFit connector to support standardized enteral connections.
- An anti-IV connector to help reduce the risk of misconnections.
- DEHP-free construction.
- Centimeter markings to at least 32 cm for insertion guidance.
- Bold centimeter indicators to help confirm placement.
- Unique tethered plug closure designed to limit exposure to ambient air.
- Soft medical-grade polymers developed to support developmental care and clinical performance.
- Wave-pattern closure plug that allows airflow through the hub to help reduce bacterial formation.
- Bottomless hub design that limits the accumulation of HBM or formula.
- Cost-effective configuration validated for indwell time up to thirty days to help reduce repeat placements.
- Open the distal tip without sharp edges or hidden cavities to help limit residual colonization.
- Three offset side ports are precision drilled to deliver smooth edges compared to large cut openings.
Intended Users: This device is designed for use by trained healthcare professionals.
Patient Target Group: This device is intended for patients of all age groups requiring nasogastric or orogastric enteral feeding.
Indications for Use: This product is indicated for neonatal and pediatric patients to provide nutrition via nasal or oral gastric placement and is not intended for use beyond 30 days.
Contraindications: This device is designed to connect only with ENFit-compatible connectors. It is not intended for use longer than 30 days or for patients with congenital gastrointestinal anomalies above the stomach requiring surgical intervention.
Device Description / Intended Purpose: The NEOMED Enteral Feeding Tube is intended for patients who require intermittent or continuous enteral nutrition via the nasogastric or orogastric pathway. Material options include polyurethane. Refer to product packaging for product-specific information.
Residual Risks and Side Effects: While outcomes vary depending on the clinical procedure, mitigations have been implemented to help minimize the following potential risks associated with NEOMED usage.
Nasogastric Feeding Tubes and associated NEOMED Extension Sets: abnormal reproductive development, allergic or systemic toxic reaction, over or under delivery of medication or nutrition, skin or tissue irritation involving the trachea, vocal cords, or gastrointestinal tract, infection, fever, sepsis, nausea, vomiting, bloating, diarrhea, delayed or prolonged therapy, pneumothorax, choking, aspiration, peritonitis, gastroenteritis, laceration, pain, and potential long-term carcinogenic or mutagenic effects.
MR Information
MR Safe
The NEOMED Enteral Feeding Tube contains only nonconducting, nonmetallic, and nonmagnetic components and presents no known hazards in MR imaging environments.
Clinical Benefits: Nasogastric and/or orogastric feeding tubes facilitate delivery of nutrition, fluids, and medications to the stomach through the nose or mouth.
Device Lifetime: Device performance has been validated for use up to 30 days.
Recommended Placement Procedure
Insertion Measurement: measure from the tip of the nose to the earlobe and then from the earlobe to midway between the xyphoid process and the umbilicus. Centimeter markings assist with placement.
A strip of sterile tape may be placed at the intended insertion depth for reference.
Insert the tube slowly into the mouth or nose, allowing the infant to swallow as advancement continues.
Advance to the predetermined length.
Precautions: Avoid unintended tracheal placement or insufficient insertion that leaves the proximal side port outside the stomach. Secure the tube before checking placement.
Verify Feeding Tube Placement: Confirm and document positioning per facility protocol, such as chest radiograph or aspiration with pH testing. Reposition or remove if uncertain.
Recommended Maintenance
Inspect tube position before each administration using catheter markings.
Verify placement per hospital protocol.
Flush with sterile water before and after administration.
Seal with tethered closure when not in use.
Recommended Feeding Tube Removal
Flush with 0.5cc–1cc sterile water.
Plug or clamp during withdrawal to help minimize aspiration risk.
Withdraw gently and discard according to institutional or regulatory requirements.
Document removal.
Disposal: Follow local, state, and national regulations for disposal of potentially biohazardous components and accessories.
Specifications
| Product Code (SKU) | Diameter (Fr) | Inner Diameter (mm) | Length (cm) | Primary Product Color |
| FTL5.0P-NC | 5 | 0.9 | 90 | Orange |
| FTL6.5P-NC | 6.5 | 1.25 | 90 | Orange |
| FTL8.0P-NC | 8 | 1.6 | 90 | Orange |
| FTM10.0P-NC | 10 | 2.45 | 60 | Orange |
| FTM12.0P-NC | 12 | 2.65 | 60 | Orange |
| FTM5.0P-NC | 5 | 0.9 | 60 | Orange |
| FTM6.5P-NC | 6.5 | 1.25 | 60 | Orange |
| FTM8.0P-NC | 8 | 1.6 | 60 | Orange |
| FTS4.0P-NC | 4 | 0.6 | 40 | Orange |
| FTS5.0P-NC | 5 | 0.9 | 40 | Orange |
| FTS6.5P-NC | 6.5 | 1.25 | 40 | Orange |
| FTS8.0P-NC | 8 | 1.6 | 40 | Orange |
| FTXL5.0P-NC | 5 | 0.9 | 105 | Orange |
| FTXL8.0P-NC | 8 | 1.6 | 105 | Orange |
| PFTL5.0P-NC | 5 | 0.9 | 90 | Purple |
| PFTL6.5P-NC | 6.5 | 1.25 | 90 | Purple |
| PFTL8.0P-NC | 8 | 1.6 | 90 | Purple |
| PFTM10.0P-NC | 10 | 2.45 | 60 | Purple |
| PFTM12.0P-NC | 12 | 2.65 | 60 | Purple |
| PFTM5.0P-NC | 5 | 0.9 | 60 | Purple |
| PFTM6.5P-NC | 6.5 | 1.25 | 60 | Purple |
| PFTM8.0P-NC | 8 | 1.6 | 60 | Purple |
| PFTS4.0P-NC | 4 | 0.6 | 40 | Purple |
| PFTS5.0P-NC | 5 | 0.9 | 40 | Purple |
| PFTS6.5P-NC | 6.5 | 1.25 | 40 | Purple |
| PFTS8.0P-NC | 8 | 1.6 | 40 | Purple |
| PFTXL5.0P-NC | 5 | 0.9 | 105 | Purple |
| PFTXL8.0P-NC | 8 | 1.6 | 105 | Purple |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Resources
Starts From
