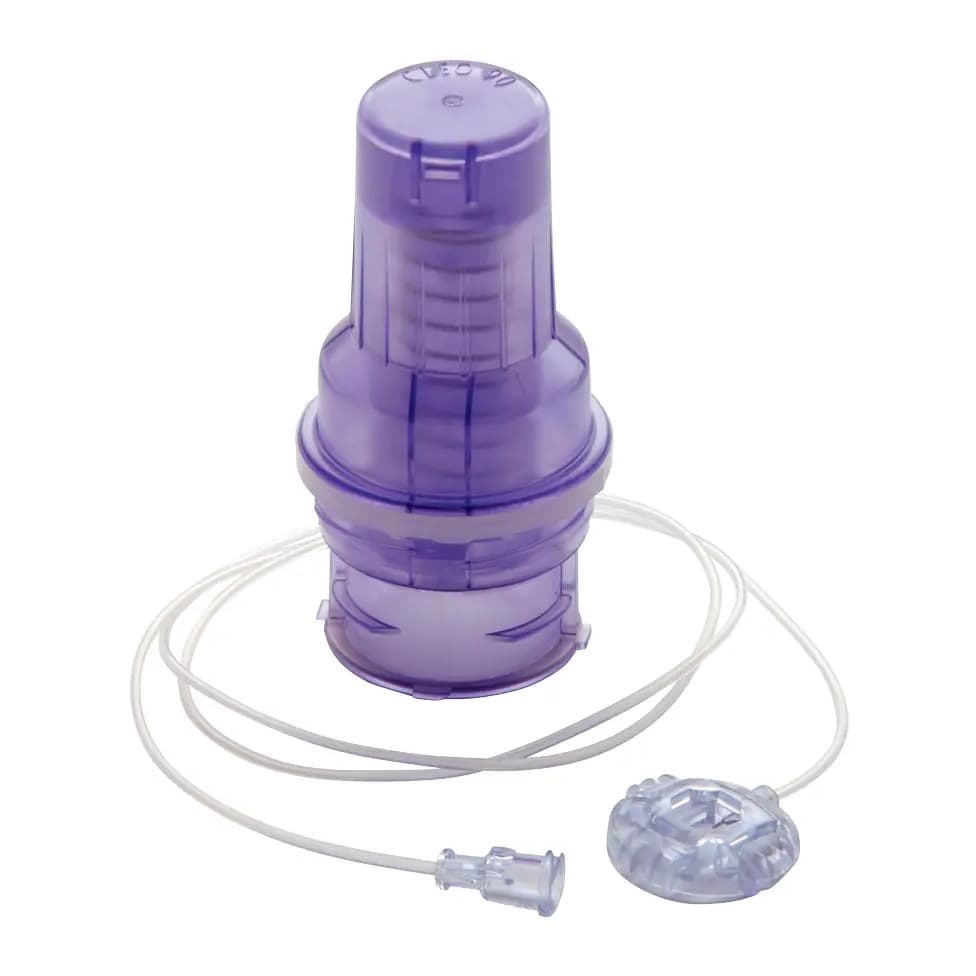
Surecan Safety II Huber Needle Infusion Set, 20 Gauge, 1/2 Inch, Y-site Injection Port - Case of 100
Earn Loyalty Points
+706Buy more to unlock extra rewards!
PayPal Pay Later
Free Shipping
Description
Surecan Safety II is the power injectable port access needle with a safety mechanism designed to reduce the risk of needlestick injuries. Its small size and unique design are intended to enhance the clinician experience in a healthcare setting and for ease of use at home.
Features
- Y-site configuration
- Tubing length cannula to Y-site: 90 +/- 10 mm
- Tubing length Y-site to connector: 82 +/- 10 mm
User Safety
- Green dot clearly shows safety mechanism activation.
- Easy to deploy safety mechanism designed to reduce needlestick injuries.
Patient Comfort
- Low profile and foam pad.
Handling
- Flexible and ergonomic wings provide secure handling.
Power Injections
- Suitable for power injections up to 325 psi.
| Model No: | Cannula diameter (mm): | Cannula Length (mm): | Cannula Length (in.): | Size: |
| 4447045-02 | 1.1 | 15 | 0.6 | 19G |
| 4447046-02 | 1.1 | 20 | 0.8 | 19G |
| 4447047-02 | 1.1 | 25 | 1.0 | 19G |
| 4447058-02 | 0.9 | 12 | 0.5 | 20G |
| 4447050-02 | 0.9 | 15 | 0.6 | 20G |
| 4447051-02 | 0.9 | 20 | 0.8 | 20G |
| 4447052-02 | 0.9 | 25 | 1.0 | 20G |
| 4447059-02 | 0.7 | 12 | 0.5 | 22G |
| 4447055-02 | 0.7 | 20 | 0.8 | 22G |
| 4447056-02 | 0.7 | 25 | 1.0 | 22G |
Specifications
| Brand | Surecan Safety II |
| Manufacturer | B. Braun |
| Application | Huber Needle Infusion Set |
| Connection Type | Luer Lock Connector |
| Flow Control Type | Pinch Clamp |
| Hub Material | Plastic / Foam |
| Hub Type | Winged Hub |
| Needle Material | Stainless Steel Needle |
| Needle Style | Standard Bevel Needle |
| Port Type | Needleless Y-Site Injection Port |
| Safety Feature | Safety Shield |
| Sterility | Sterile |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
FAQs
Does Surecan Safety II contain DEHP or latex?
Surecan Safety II needles are not manufactured with DEHP or natural rubber latex.
Can I use the Surecan Safety II needle with a power injector?
Yes, all the Surecan Safety II needles can be used for power injection.
Note: The Surecan Safety II needle with CARESITE requires injection through the CARESITE valve, and not through the distal end of the tubing.
Are the Surecan Safety II needles MRI conditional?
Yes, Surecan Safety II needles are MRI conditional (See product IFU).
The IFU and labels state the Surecan Safety II needles can be used for power injection up to 325 psi. Why does the clamp on the Y-Site configurations show 45 psi?
Power injection up to 325 PSI should always be applied through the CARESITE valve at the Y-Site. Forty five (45) PSI is the maximum pressure allowed through the injection port at the distal end of the infusion set.
How long can a Surecan Safety II needle be left in the port?
Surecan Safety II needles may be used for up to seven days, in the absence of infection, redness, swelling, or pain, and in accordance with institutional protocol.
How can I prevent the base plate from sliding down the needle during port access?
The base plate is secured by a small retention plate under the wings. Grasp the wings and fold vertically to prevent unintentional unclipping of the base plate. Grasp the wings to prepare for insertion. Keep fingers off of the base plate. Remove the needle guard with the other hand.
Do you have any suggestions for dressing the Surecan Safety II needle?
Dress the device per institutional protocol. B. Braun suggests placing two adhesive strips over the wings and one over the extension set. Apply a clear dressing over the entire Port Access needle in a manner to prevent tenting.
What is the gravity flow rate for the Surecan Safety II needle?
| Gauge | Needle Length | NaCl mL/min1 (average) |
| 19G | 20 mm (0.8 in) | 38.9 |
| 19G | 25 mm (1.0 in) | 38.6 |
| 19G | 38 mm (1.5 in) | 35.5 |
| 20G | 20 mm (0.8 in) | 20.2 |
| 20G | 25 mm (1.0 in) | 21.5 |
| 20G | 38 mm (1.5 in) | 21.3 |
| 22G | 15 mm (0.6 in) | 8.9 |
| 22G | 25 mm (1.0 in) | 7.7 |
| 22G | 32 mm (1.3 in) | 7.8 |
Can I use the Bio-Patch dressing with the Surecan Safety II Needle?
Yes, Bio-Patch can be used with Surecan Safety II needles. Follow the Bio-Patch manufacturer�s IFU.
Are the Surecan Safety II Port Access Needles siliconized?
No. Like other port access needles, there is no silicone on the needles.
Removing occlusive dressings can be difficult. Do you have any recommendations?
Support the Surecan Safety II needle with one hand while removing the dressing.
How is Surecan Safety II sterilized?
Surecan Safety II is sterilized by ethylene oxide (also known as EO or EtO).
Description
Surecan Safety II is the power injectable port access needle with a safety mechanism designed to reduce the risk of needlestick injuries. Its small size and unique design are intended to enhance the clinician experience in a healthcare setting and for ease of use at home.
Features
- Y-site configuration
- Tubing length cannula to Y-site: 90 +/- 10 mm
- Tubing length Y-site to connector: 82 +/- 10 mm
User Safety
- Green dot clearly shows safety mechanism activation.
- Easy to deploy safety mechanism designed to reduce needlestick injuries.
Patient Comfort
- Low profile and foam pad.
Handling
- Flexible and ergonomic wings provide secure handling.
Power Injections
- Suitable for power injections up to 325 psi.
| Model No: | Cannula diameter (mm): | Cannula Length (mm): | Cannula Length (in.): | Size: |
| 4447045-02 | 1.1 | 15 | 0.6 | 19G |
| 4447046-02 | 1.1 | 20 | 0.8 | 19G |
| 4447047-02 | 1.1 | 25 | 1.0 | 19G |
| 4447058-02 | 0.9 | 12 | 0.5 | 20G |
| 4447050-02 | 0.9 | 15 | 0.6 | 20G |
| 4447051-02 | 0.9 | 20 | 0.8 | 20G |
| 4447052-02 | 0.9 | 25 | 1.0 | 20G |
| 4447059-02 | 0.7 | 12 | 0.5 | 22G |
| 4447055-02 | 0.7 | 20 | 0.8 | 22G |
| 4447056-02 | 0.7 | 25 | 1.0 | 22G |
Specifications
| Brand | Surecan Safety II |
| Manufacturer | B. Braun |
| Application | Huber Needle Infusion Set |
| Connection Type | Luer Lock Connector |
| Flow Control Type | Pinch Clamp |
| Hub Material | Plastic / Foam |
| Hub Type | Winged Hub |
| Needle Material | Stainless Steel Needle |
| Needle Style | Standard Bevel Needle |
| Port Type | Needleless Y-Site Injection Port |
| Safety Feature | Safety Shield |
| Sterility | Sterile |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
FAQs
Does Surecan Safety II contain DEHP or latex?
Surecan Safety II needles are not manufactured with DEHP or natural rubber latex.
Can I use the Surecan Safety II needle with a power injector?
Yes, all the Surecan Safety II needles can be used for power injection.
Note: The Surecan Safety II needle with CARESITE requires injection through the CARESITE valve, and not through the distal end of the tubing.
Are the Surecan Safety II needles MRI conditional?
Yes, Surecan Safety II needles are MRI conditional (See product IFU).
The IFU and labels state the Surecan Safety II needles can be used for power injection up to 325 psi. Why does the clamp on the Y-Site configurations show 45 psi?
Power injection up to 325 PSI should always be applied through the CARESITE valve at the Y-Site. Forty five (45) PSI is the maximum pressure allowed through the injection port at the distal end of the infusion set.
How long can a Surecan Safety II needle be left in the port?
Surecan Safety II needles may be used for up to seven days, in the absence of infection, redness, swelling, or pain, and in accordance with institutional protocol.
How can I prevent the base plate from sliding down the needle during port access?
The base plate is secured by a small retention plate under the wings. Grasp the wings and fold vertically to prevent unintentional unclipping of the base plate. Grasp the wings to prepare for insertion. Keep fingers off of the base plate. Remove the needle guard with the other hand.
Do you have any suggestions for dressing the Surecan Safety II needle?
Dress the device per institutional protocol. B. Braun suggests placing two adhesive strips over the wings and one over the extension set. Apply a clear dressing over the entire Port Access needle in a manner to prevent tenting.
What is the gravity flow rate for the Surecan Safety II needle?
| Gauge | Needle Length | NaCl mL/min1 (average) |
| 19G | 20 mm (0.8 in) | 38.9 |
| 19G | 25 mm (1.0 in) | 38.6 |
| 19G | 38 mm (1.5 in) | 35.5 |
| 20G | 20 mm (0.8 in) | 20.2 |
| 20G | 25 mm (1.0 in) | 21.5 |
| 20G | 38 mm (1.5 in) | 21.3 |
| 22G | 15 mm (0.6 in) | 8.9 |
| 22G | 25 mm (1.0 in) | 7.7 |
| 22G | 32 mm (1.3 in) | 7.8 |
Can I use the Bio-Patch dressing with the Surecan Safety II Needle?
Yes, Bio-Patch can be used with Surecan Safety II needles. Follow the Bio-Patch manufacturer�s IFU.
Are the Surecan Safety II Port Access Needles siliconized?
No. Like other port access needles, there is no silicone on the needles.
Removing occlusive dressings can be difficult. Do you have any recommendations?
Support the Surecan Safety II needle with one hand while removing the dressing.
How is Surecan Safety II sterilized?
Surecan Safety II is sterilized by ethylene oxide (also known as EO or EtO).