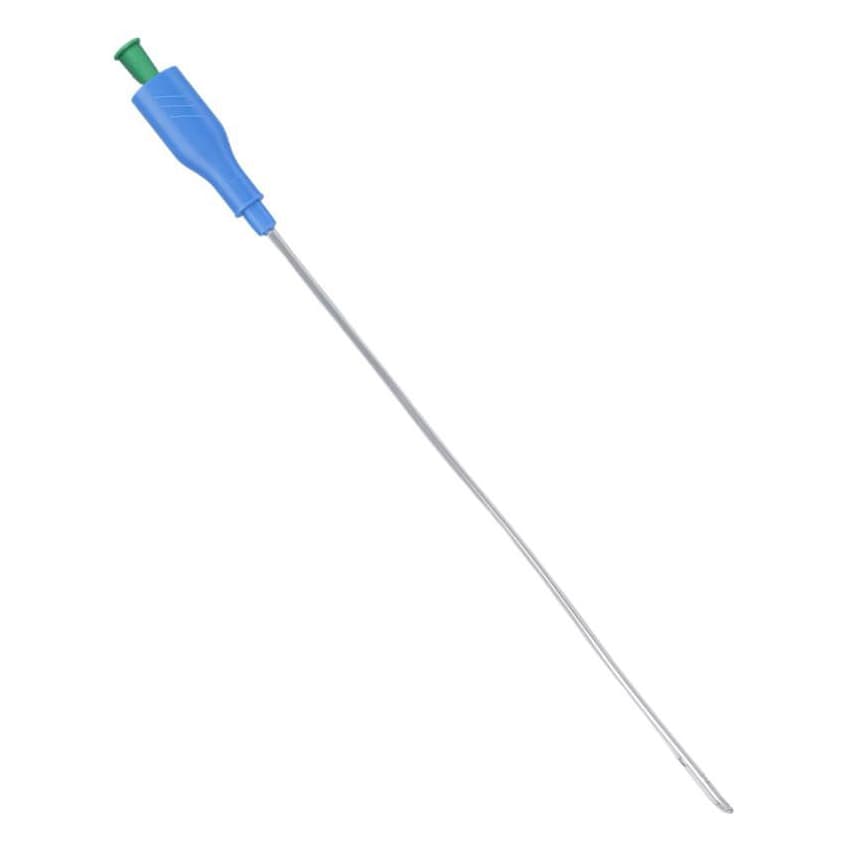
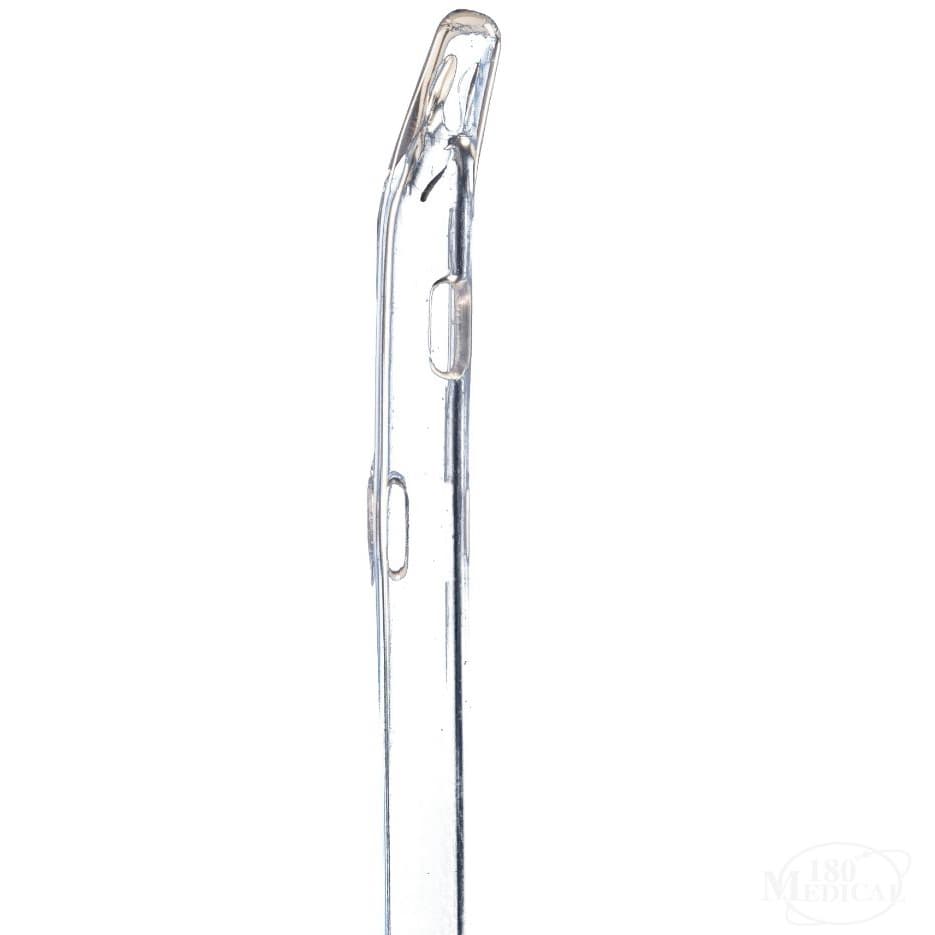

TruCath Oasis Ready-to-Use Hydrophilic Intermittent Catheter, Coude Tip
Starts From
Description
TruCath® Oasis™ Ready-to-Use Hydrophilic Intermittent Catheters are designed to provide a convenient, comfortable catheterization experience without added preparation steps. Ready to use straight from the package, these catheters require no additional water or lubricant, helping save time while reducing mess. The hydrophilic coating is pre-hydrated to create a smooth, low-friction surface that supports gentle insertion and enhanced comfort during use. Each catheter is sterile and individually packaged for hygienic, single-use application.
Engineered with a coudé tip, TruCath® Oasis™ catheters are slightly curved to assist with maneuvering around urethral obstructions or enlarged prostate anatomy. The TruProtect™ grip supports a no-touch technique to help minimize the risk of cross-contamination, while easy-open packaging with a ring-pull and adhesive tab enhances usability and convenience. Free from natural rubber latex, DEHP, BPA, and other phthalates, these catheters offer a safe and reliable option for intermittent catheterization.
Features:
- Length: 16 Inch
- Ready-to-Use Hydrophilic Design: Pre-hydrated coating requires no water or lubricant, saving time and reducing mess.
- Hydrophilic Coating: Creates a silky, smooth surface to reduce friction and enhance comfort during insertion and removal.
- Coudé Tip: Slightly curved tip helps navigate around urethral obstructions and blockages.
- TruProtect™ No-TouchGrip: Supports hygienic handling to help reduce the risk of cross-contamination.
- Easy-Open Packaging: Ring-pull opening and adhesive hanging tab improve ease of use and accessibility.
- Male Length Catheter: 16-inch length designed for male anatomy.
- Latex- and Phthalate-Free: Not made with natural rubber latex, DEHP, BPA, or other phthalates.
- Sterile, Single-Use: Individually packaged for safe, hygienic, one-time use.
Indications For Use:
The TruCath® Intermittent Catheters are indicated for intermittent urinary catheterization by inserting through the urethra to pass urine from the bladder.
Contraindications:
- Acute urethritis
- Acute prostatitis
- Acute epididymitis
- Patients with a PVC or Gel allergy
- Patients with calcareous urolithiasis
Warnings:
- After removing the catheter from the packaging, do not wipe or scrub the surface of the catheter by either physical force or chemicals to avoid damaging the hydrophilic coating which may result in efficacy loss.
- This is a single use device.
- Do not re-sterilize any portion of this device.
- Reuse and/or repackaging may create a risk of patient or user infection, compromise the structural integrity and/or essential material and design characteristics of the device, which may lead to device failure, and/or lead to injury, illness or death of the patient.
Precautions:
- This product can be used in healthcare centers under supervision of appropriately qualified and trained personnel or at home by patients according to the instructions for use.
- To prevent urinary tract infection, appropriate aseptic steps should be taken to areas around the urethral opening prior to insertion.
- This product is disposable, do not reuse.
- Do not use if the package is damaged, contaminated, or expired.
- Special attention shall be taken for patients with disturbance of consciousness as involuntary pull of the catheter may lead to bladder and urethra mucosal injury or break the catheter.
- Intubation and extubation should be as gentle as possible to prevent urethral injury.
- If the catheter is applied with a guide wire, the operator shall ensure that the guide wire is at the foremost end of the catheter. Pumping action shall be avoided as the guide wire may pass through the eyelets with the risk of injuring the urethral mucosa.
- Slowly advance the catheter through the urethra into the bladder. If substantial resistance is met, do not force the catheter. Slowly pull out the catheter and look for reasons.
- If there is no urine draining into the bag, take the following steps: Check for and remove any kinks in the catheter or the drainage bag tubing. Check the position of the catheter and drainage bag. Ensure the bag is positioned below the bladder when lying, sitting or standing.
- For overfilled bladder, the urine should not be discharged too fast which may lead to shock or bladder bleeding.
- Catheter insertion should always be carried out in accordance with local and national best practice policies by appropriately qualified and trained personnel.
Specifications
| Brand | TruCath Oasis |
| Manufacturer | HR Healthcare |
| Application | Urethral Catheter |
| Eyes | 2 Opposing Polished Eyelets |
| Gender | Male |
| Hydrophilic Activation Method | Ready-to-Use Hydrophilic |
| Length | 16 Inch |
| Material | Hydrophilic-Coated PVC |
| Sterility | Sterile |
| Style | Coudé Tip |
| Usage | Disposable |
| User | Adult |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Resources
Description
TruCath® Oasis™ Ready-to-Use Hydrophilic Intermittent Catheters are designed to provide a convenient, comfortable catheterization experience without added preparation steps. Ready to use straight from the package, these catheters require no additional water or lubricant, helping save time while reducing mess. The hydrophilic coating is pre-hydrated to create a smooth, low-friction surface that supports gentle insertion and enhanced comfort during use. Each catheter is sterile and individually packaged for hygienic, single-use application.
Engineered with a coudé tip, TruCath® Oasis™ catheters are slightly curved to assist with maneuvering around urethral obstructions or enlarged prostate anatomy. The TruProtect™ grip supports a no-touch technique to help minimize the risk of cross-contamination, while easy-open packaging with a ring-pull and adhesive tab enhances usability and convenience. Free from natural rubber latex, DEHP, BPA, and other phthalates, these catheters offer a safe and reliable option for intermittent catheterization.
Features:
- Length: 16 Inch
- Ready-to-Use Hydrophilic Design: Pre-hydrated coating requires no water or lubricant, saving time and reducing mess.
- Hydrophilic Coating: Creates a silky, smooth surface to reduce friction and enhance comfort during insertion and removal.
- Coudé Tip: Slightly curved tip helps navigate around urethral obstructions and blockages.
- TruProtect™ No-TouchGrip: Supports hygienic handling to help reduce the risk of cross-contamination.
- Easy-Open Packaging: Ring-pull opening and adhesive hanging tab improve ease of use and accessibility.
- Male Length Catheter: 16-inch length designed for male anatomy.
- Latex- and Phthalate-Free: Not made with natural rubber latex, DEHP, BPA, or other phthalates.
- Sterile, Single-Use: Individually packaged for safe, hygienic, one-time use.
Indications For Use:
The TruCath® Intermittent Catheters are indicated for intermittent urinary catheterization by inserting through the urethra to pass urine from the bladder.
Contraindications:
- Acute urethritis
- Acute prostatitis
- Acute epididymitis
- Patients with a PVC or Gel allergy
- Patients with calcareous urolithiasis
Warnings:
- After removing the catheter from the packaging, do not wipe or scrub the surface of the catheter by either physical force or chemicals to avoid damaging the hydrophilic coating which may result in efficacy loss.
- This is a single use device.
- Do not re-sterilize any portion of this device.
- Reuse and/or repackaging may create a risk of patient or user infection, compromise the structural integrity and/or essential material and design characteristics of the device, which may lead to device failure, and/or lead to injury, illness or death of the patient.
Precautions:
- This product can be used in healthcare centers under supervision of appropriately qualified and trained personnel or at home by patients according to the instructions for use.
- To prevent urinary tract infection, appropriate aseptic steps should be taken to areas around the urethral opening prior to insertion.
- This product is disposable, do not reuse.
- Do not use if the package is damaged, contaminated, or expired.
- Special attention shall be taken for patients with disturbance of consciousness as involuntary pull of the catheter may lead to bladder and urethra mucosal injury or break the catheter.
- Intubation and extubation should be as gentle as possible to prevent urethral injury.
- If the catheter is applied with a guide wire, the operator shall ensure that the guide wire is at the foremost end of the catheter. Pumping action shall be avoided as the guide wire may pass through the eyelets with the risk of injuring the urethral mucosa.
- Slowly advance the catheter through the urethra into the bladder. If substantial resistance is met, do not force the catheter. Slowly pull out the catheter and look for reasons.
- If there is no urine draining into the bag, take the following steps: Check for and remove any kinks in the catheter or the drainage bag tubing. Check the position of the catheter and drainage bag. Ensure the bag is positioned below the bladder when lying, sitting or standing.
- For overfilled bladder, the urine should not be discharged too fast which may lead to shock or bladder bleeding.
- Catheter insertion should always be carried out in accordance with local and national best practice policies by appropriately qualified and trained personnel.
Specifications
| Brand | TruCath Oasis |
| Manufacturer | HR Healthcare |
| Application | Urethral Catheter |
| Eyes | 2 Opposing Polished Eyelets |
| Gender | Male |
| Hydrophilic Activation Method | Ready-to-Use Hydrophilic |
| Length | 16 Inch |
| Material | Hydrophilic-Coated PVC |
| Sterility | Sterile |
| Style | Coudé Tip |
| Usage | Disposable |
| User | Adult |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Resources
Starts From
