Your Cart
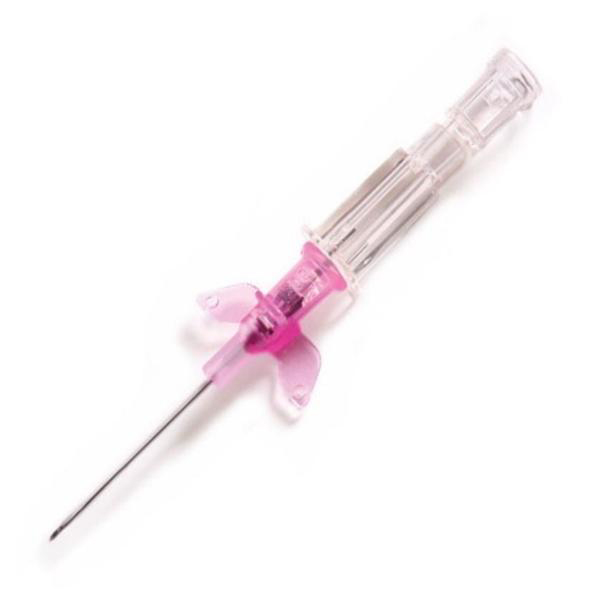
B. Braun Introcan Safety Peripheral IV Catheter, Polyurethane, 20 Gauge, 1.25 Inch - Each
You will earn up to 6 reward points!
You will earn upto 6 reward points!
Description
Sizing
Resources
Warranty
Return
B.Braun unique catheters have a passive safety device that is automatically activated and cannot be bypassed. Easy to use and features double flashback technology for successful needle placement every time.
Features
- Universal bevel needle.
- Passive safety device.
- Winged configurations.
- Material: Polyurethane
- Double flashback technology.
- Needle flash confirms needle is in vein.
- Catheter flash confirms catheter placement.
Passive Safety technology is preferred
Introcan Safety IV Catheter‘s provide safety mechanism deploys without user activation. Unnecessary injuries may occur with active safety devices because activation is often delayed or never happens. That’s why designed the B. Braun Introcan Safety IV Catheter and why it’s raising the standard in needlestick prevention.
Fully automatic passive safety that cannot be bypassed
Passive Safety helps clinicians meet the INS Standards of Practice and is proven to be two times better than a semi-automatic ‘push-button’ safety shield and three times better than a manually sliding shield.
The Passive Safety Shield
The shield automatically covers the needle tip upon needle withdrawal with no need for user activation.
Facilitates best practice
Designed to prevent accidental needlesticks
Fully Automatic Passive Safety Shield does not require manual activation, CANNOT BE BYPASSED and prevents needle reinsertions when activated.
Designed to promote easy access for catheter insertion
Universal Bevel Needle has a unique back-cut design that is designed to produce a tricuspid incision for insertion. It also is designed for a wide variety of insertion angles for deep and superficial vascular access.
Universal Bevel - Designed to allow flexibility of insertion angles.
Designed to promote first-stick success
Double Flashback Technology is designed to provide a quick visual confirmation of vein puncture with separate needle and catheter flashback.
Designed to enable longer in-dwelling
Catheters are available in polyurethane (PUR) which provides softer in-dwelling performance. Introcan Safety IV Catheters are not made with DEHP or natural rubber latex.
Removable flashplug: The removable vented flashplug permits attachment of a syringe.
Introcan Safety IV Catheters help you cut costs and “go green”
- Generate less waste with smaller, lighter components
- Designed to save money and avoid throwing away unused components (compared to integrated catheters)
- Helps to cut costs by reducing needlesticks, materials, and waste
Quick product identification: Each box label carries an illustration of the product inside so the contents can be verified before opening.
Easy to open: The openings of box lids and peel packaging are clearly indicated with a green B. Braun stripe.
Simple gauge identification: Color-coded labels for easy gauge size identification.
| Gauge x Needle Length | Gravity flow rate mL/min (300 psi) |
| 24G x 0.55 inch | 26 |
| 24G x 0.75 inch | 22 |
| 22G x 1 inch | 35 |
| 22G x 1.75 inch | 26 |
| 20G x 1 inch | 65 |
| 20G x 1.25 inch | 60 |
| 20G x 1.75 inch | 57 |
| 18G x 1.25 inch | 105 |
| 18G x 1.75 inch | 100 |
| 18G x 2.5 inch | 85 |
| 16G x 1.25 inch | 215 |
| 16G x 2 inch | 210 |
| 14G x 1.25 inch | 350 |
| 14G x 2 inch | 345 |
| Brand | Introcan Safety |
| Manufacturer | B. Braun |
| Application | Peripheral IV Catheter |
| Blood Control | Non Blood Control |
| Catheter Material | Polyurethane |
| Usage | Disposable |
| Hub Material | Plastic Hub |
| Sterility | Sterile |
| Safety Feature | Sliding |
| Vessel Entry Confirmation | Double Flashback Technology |
| X-Ray Compatibility | Radiopaque |
| Safety Activation | Passive Safety |
| Pressure Rating | 300 psi |
| Number of Ports | Without Port |
| Needle Point Style | Bevel Needle |
| Needle Material | Stainless Steel |
| Hub Type | Winged Hub |
| DEHP Indicator | Not Made With DEHP |
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
B.Braun unique catheters have a passive safety device that is automatically activated and cannot be bypassed. Easy to use and features double flashback technology for successful needle placement every time.
Features
- Universal bevel needle.
- Passive safety device.
- Winged configurations.
- Material: Polyurethane
- Double flashback technology.
- Needle flash confirms needle is in vein.
- Catheter flash confirms catheter placement.
Passive Safety technology is preferred
Introcan Safety IV Catheter‘s provide safety mechanism deploys without user activation. Unnecessary injuries may occur with active safety devices because activation is often delayed or never happens. That’s why designed the B. Braun Introcan Safety IV Catheter and why it’s raising the standard in needlestick prevention.
Fully automatic passive safety that cannot be bypassed
Passive Safety helps clinicians meet the INS Standards of Practice and is proven to be two times better than a semi-automatic ‘push-button’ safety shield and three times better than a manually sliding shield.
The Passive Safety Shield
The shield automatically covers the needle tip upon needle withdrawal with no need for user activation.
Facilitates best practice
Designed to prevent accidental needlesticks
Fully Automatic Passive Safety Shield does not require manual activation, CANNOT BE BYPASSED and prevents needle reinsertions when activated.
Designed to promote easy access for catheter insertion
Universal Bevel Needle has a unique back-cut design that is designed to produce a tricuspid incision for insertion. It also is designed for a wide variety of insertion angles for deep and superficial vascular access.
Universal Bevel - Designed to allow flexibility of insertion angles.
Designed to promote first-stick success
Double Flashback Technology is designed to provide a quick visual confirmation of vein puncture with separate needle and catheter flashback.
Designed to enable longer in-dwelling
Catheters are available in polyurethane (PUR) which provides softer in-dwelling performance. Introcan Safety IV Catheters are not made with DEHP or natural rubber latex.
Removable flashplug: The removable vented flashplug permits attachment of a syringe.
Introcan Safety IV Catheters help you cut costs and “go green”
- Generate less waste with smaller, lighter components
- Designed to save money and avoid throwing away unused components (compared to integrated catheters)
- Helps to cut costs by reducing needlesticks, materials, and waste
Quick product identification: Each box label carries an illustration of the product inside so the contents can be verified before opening.
Easy to open: The openings of box lids and peel packaging are clearly indicated with a green B. Braun stripe.
Simple gauge identification: Color-coded labels for easy gauge size identification.
| Gauge x Needle Length | Gravity flow rate mL/min (300 psi) |
| 24G x 0.55 inch | 26 |
| 24G x 0.75 inch | 22 |
| 22G x 1 inch | 35 |
| 22G x 1.75 inch | 26 |
| 20G x 1 inch | 65 |
| 20G x 1.25 inch | 60 |
| 20G x 1.75 inch | 57 |
| 18G x 1.25 inch | 105 |
| 18G x 1.75 inch | 100 |
| 18G x 2.5 inch | 85 |
| 16G x 1.25 inch | 215 |
| 16G x 2 inch | 210 |
| 14G x 1.25 inch | 350 |
| 14G x 2 inch | 345 |
| Brand | Introcan Safety |
| Manufacturer | B. Braun |
| Application | Peripheral IV Catheter |
| Blood Control | Non Blood Control |
| Catheter Material | Polyurethane |
| Usage | Disposable |
| Hub Material | Plastic Hub |
| Sterility | Sterile |
| Safety Feature | Sliding |
| Vessel Entry Confirmation | Double Flashback Technology |
| X-Ray Compatibility | Radiopaque |
| Safety Activation | Passive Safety |
| Pressure Rating | 300 psi |
| Number of Ports | Without Port |
| Needle Point Style | Bevel Needle |
| Needle Material | Stainless Steel |
| Hub Type | Winged Hub |
| DEHP Indicator | Not Made With DEHP |
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.

