
Starts From
Description
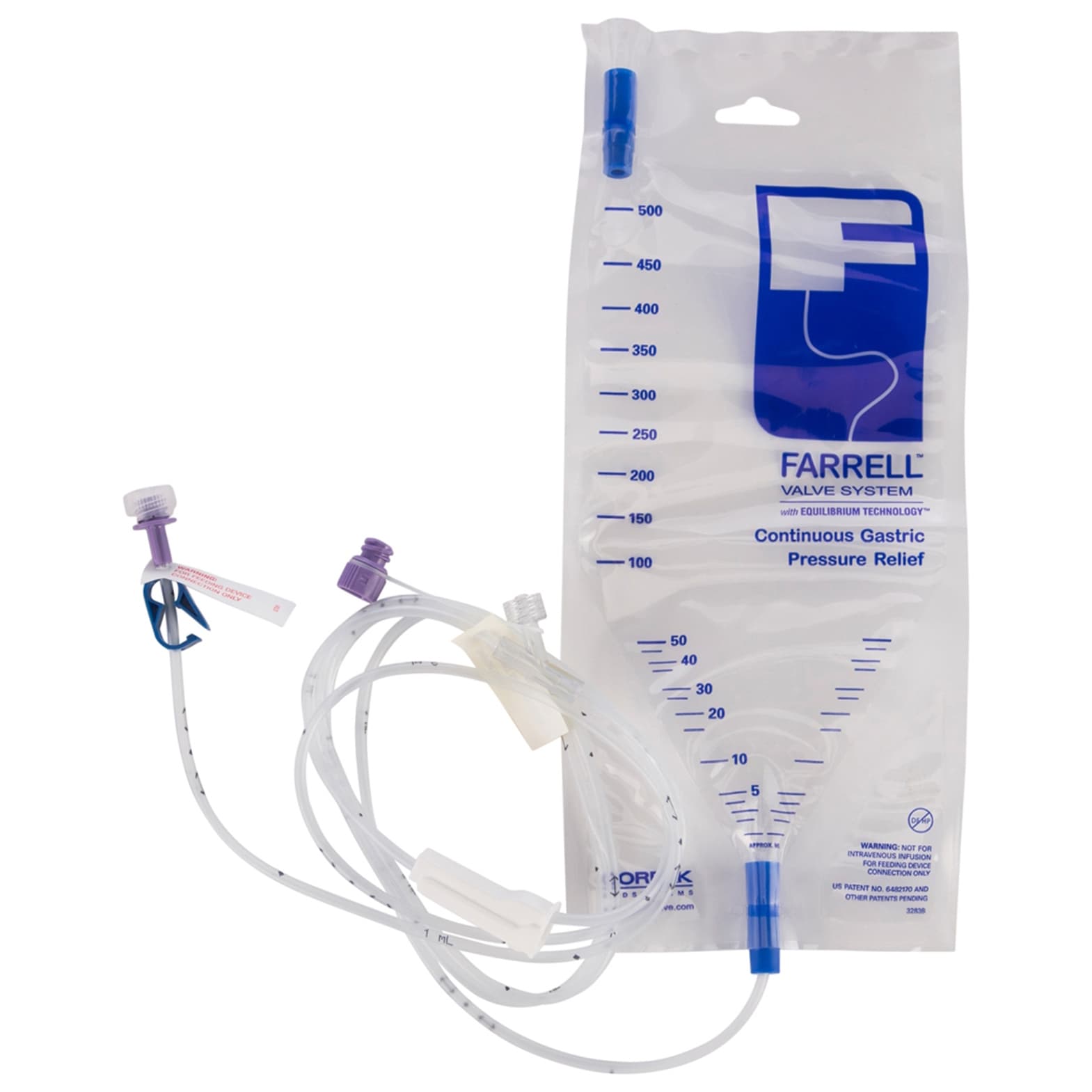
Avanos Farrell Valve Closed Enteral Decompression System is a closed reservoir system intended to evacuate excess gas (gastric distention/bloating) as well as drain/collect enteral feeding formula and gastrointestinal tract contents from patients who utilize an enteral device. The Farrell Valve System is suitable for use with infant, pediatric, and adult patients.
Features
- The Farrell Valve System has Equilibrium Technology.
- The best gastric pressure device is designed to help those who suffer from poor stomach motility, pain, and bloating.
- Maximizes enteral feeding by providing a conduit to continuously decompress the stomach, enabling it to eat at its own speed.
How to Use?
- Hang the FARRELL Bag at the same height as the feeding bag/ container.
- Close WHITE clamp above Farrell “Y” Port.
- A) Attach the enteral administration set connector to the “Y” Port on the Farrell Tubing. Note: For syringe feed, attach the syringe to the “Y” Port.
- B)Prime Farrell Tubing (3 cm) before the distal end of the Farrell Set Connector. Note: For continuous feeding, prime the pump according to the pump instructions.
- Attach the Farrell Set Connector to the feeding tube (Reminder: Confirm connection to an enteral feeding device only and NOT to an IV set.).
- Position the Farrell “Y” port at or below the patient’s stomach.
- Open the BLUE clamp to establish flow, then open the WHITE clamp. Note: The Normal height of the formula in the Farrell Tubing will be slightly above the patient’s stomach level. The formula may continuously move up and down in the Farrell Tubing.
Medication Administration
- When administering medication, use the access port on the feeding tube if possible.
- Close the BLUE clamp before opening the feeding tube access port.
- Administer medication.
- Wait 5–10 minutes before closing the feeding tube access port and re-opening the BLUE clamp.
Warning: When the Farrell Tubing line is open (Open WHITE clamp), the pump’s “occlude” alarm will not function, as the formula will continue to flow into the Farrell Bag.
| Total Farrell Line Volume | 9.6 ml |
| Priming Volume | 3.2 ml |
Specifications
| Brand | Farrell Valve |
| Manufacturer | Avanos Medical |
| Application | Farrell Valve |
| For Use With | For All enteral Feeding Devices |
| Requires Sterilization Prior to Use | False |
| Sterile | False |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Description
Avanos Farrell Valve Closed Enteral Decompression System is a closed reservoir system intended to evacuate excess gas (gastric distention/bloating) as well as drain/collect enteral feeding formula and gastrointestinal tract contents from patients who utilize an enteral device. The Farrell Valve System is suitable for use with infant, pediatric, and adult patients.
Features
- The Farrell Valve System has Equilibrium Technology.
- The best gastric pressure device is designed to help those who suffer from poor stomach motility, pain, and bloating.
- Maximizes enteral feeding by providing a conduit to continuously decompress the stomach, enabling it to eat at its own speed.
How to Use?
- Hang the FARRELL Bag at the same height as the feeding bag/ container.
- Close WHITE clamp above Farrell “Y” Port.
- A) Attach the enteral administration set connector to the “Y” Port on the Farrell Tubing. Note: For syringe feed, attach the syringe to the “Y” Port.
- B)Prime Farrell Tubing (3 cm) before the distal end of the Farrell Set Connector. Note: For continuous feeding, prime the pump according to the pump instructions.
- Attach the Farrell Set Connector to the feeding tube (Reminder: Confirm connection to an enteral feeding device only and NOT to an IV set.).
- Position the Farrell “Y” port at or below the patient’s stomach.
- Open the BLUE clamp to establish flow, then open the WHITE clamp. Note: The Normal height of the formula in the Farrell Tubing will be slightly above the patient’s stomach level. The formula may continuously move up and down in the Farrell Tubing.
Medication Administration
- When administering medication, use the access port on the feeding tube if possible.
- Close the BLUE clamp before opening the feeding tube access port.
- Administer medication.
- Wait 5–10 minutes before closing the feeding tube access port and re-opening the BLUE clamp.
Warning: When the Farrell Tubing line is open (Open WHITE clamp), the pump’s “occlude” alarm will not function, as the formula will continue to flow into the Farrell Bag.
| Total Farrell Line Volume | 9.6 ml |
| Priming Volume | 3.2 ml |
Specifications
| Brand | Farrell Valve |
| Manufacturer | Avanos Medical |
| Application | Farrell Valve |
| For Use With | For All enteral Feeding Devices |
| Requires Sterilization Prior to Use | False |
| Sterile | False |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Starts From
