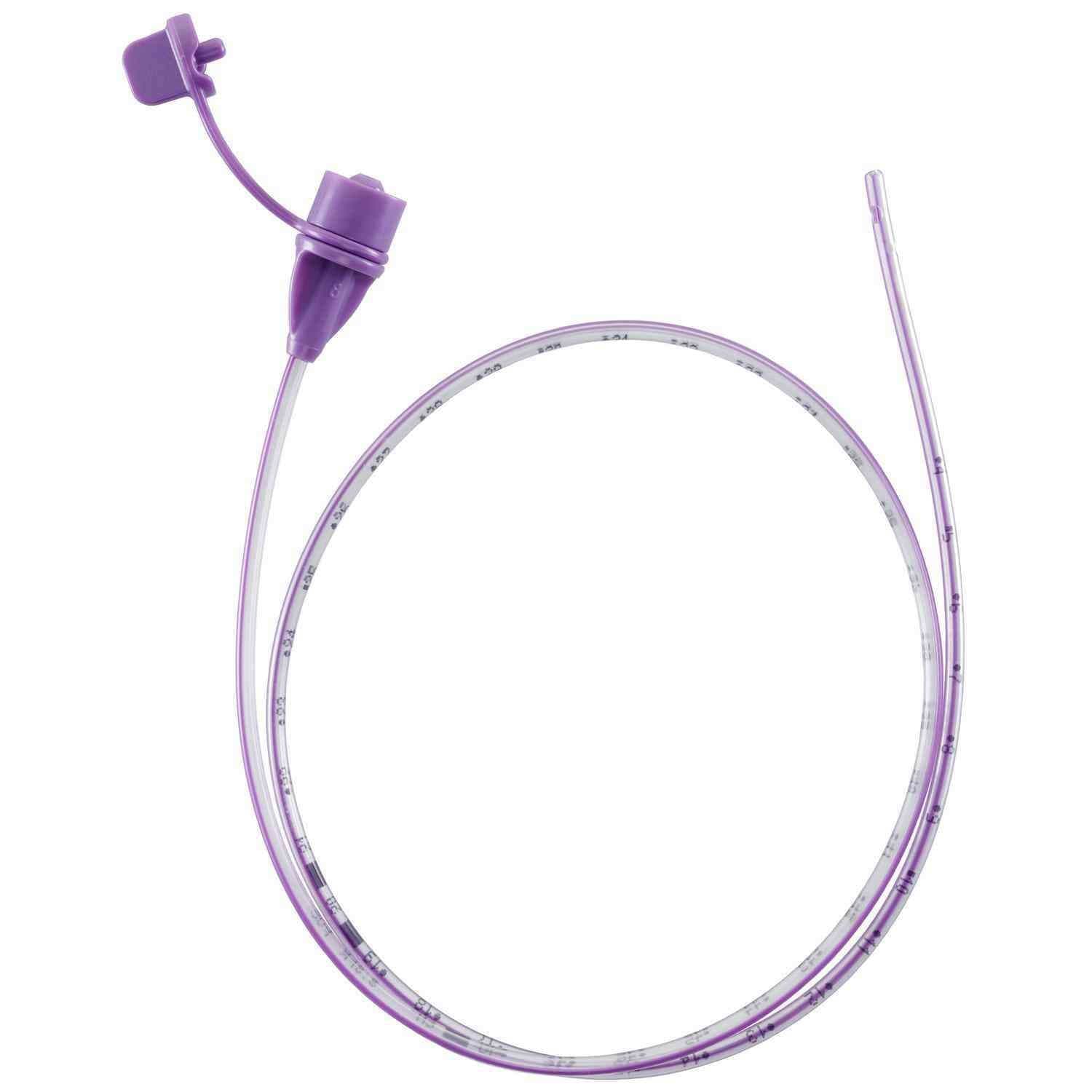

NeoMed PVC Nasogastric Feeding Tube With ENFit Connector
PayPal Pay Later
Starts From
Description
NeoMed PVC Feeding Tube With ENFit Connector is engineered to support gentle nasogastric and orogastric feeding by helping minimize tissue irritation commonly associated with oversized side ports or unfinished distal tips. The distal tip is designed to remain smooth, well formed, and open, while exposed surfaces are structured for accessibility and efficient cleaning. Attention to hub configuration and material flow pathways helps limit pooling within the distal end and recessed caps, factors that are often associated with bacterial transfer, making this feeding tube a dependable choice for neonatal and pediatric enteral nutrition in clinical environments.
Features
- BPA-free construction for added material safety.
- DEHP-free formulation to support sensitive patient populations.
- Centimeter graduations extending to at least 32 cm for accurate measurement.
- High-visibility insertion markings at each centimeter assist with placement confirmation.
- Tethered plug closure design enhances protection from ambient air exposure.
- Soft medical-grade polymers are selected to support developmental care and clinical performance.
- Wave-pattern closure plug promotes airflow through the hub, helping limit bacterial formation.
- Bottomless hub configuration reduces areas where HBM and formula could accumulate.
- Cost-effective design validated for indwell time up to thirty days to reduce repeat placements.
- Open distal tip without sharp edges or concealed cavities helps limit residual colonization.
- Three offset side ports are precision drilled to create smooth edges compared to larger cut holes used by other brands.
Intended Users: This device is designed for use by trained healthcare professionals.
Patient Target Group: This device is intended for patients of all age groups who require nasogastric or orogastric enteral feeding.
Indications for Use: This product is indicated for neonatal and pediatric patients to provide nutrition through nasal or oral gastric placement and is not intended for use beyond 30 days.
Contraindications: This device is designed to connect only with ENFit compatible connectors. It is not intended for use longer than 30 days or for patients with congenital GI tract anomalies above the stomach that require surgical intervention.
Device Description / Intended Purpose: The NEOMED Enteral Feeding Tube is intended for patients requiring intermittent or continuous enteral nutrition through nasogastric or orogastric pathways. Material options include PVC. Refer to product packaging for product-specific details.
Residual Risks and Side Effects: While outcomes vary depending on the clinical procedure, mitigations have been implemented to help minimize the following potential risks related to NEOMED usage.
Nasogastric Feeding Tubes and the associated NEOMED Extension Sets: abnormal reproductive development, allergic/systemic toxic reaction, over/under delivery of medication or nutrition, skin or tissue irritation involving the trachea, vocal cords, or gastrointestinal tract, infection, fever, sepsis, nausea, vomiting, bloating, diarrhea, delayed or prolonged therapy, pneumothorax, choking, aspiration, peritonitis, gastroenteritis, laceration, pain, and potential long-term carcinogenic or mutagenic effects.
MR Information
MR Safe
The NEOMED Enteral Feeding Tube contains only nonconducting, nonmetallic, and nonmagnetic components and presents no known hazards in MR imaging environments.
Clinical Benefits: Nasogastric and orogastric feeding tubes facilitate delivery of nutrition, fluids, and medications to the stomach through the nose or mouth.
Device Lifetime: Device performance has been validated for use up to 30 days.
Recommended Placement Procedure
Insertion Measurement: measure from the tip of the nose to the earlobe and then from the earlobe to midway between the xyphoid process and umbilicus. Centimeter markings on the catheter assist in placement.
A strip of sterile tape may be placed at the intended insertion depth for reference.
Insert the tube slowly into the mouth or nose, allowing the infant to swallow as advancement continues.
Advance to the predetermined length.
Precautions: Avoid unintended tracheal placement or insufficient insertion that leaves the proximal side hole outside the stomach. Secure the tube before checking placement.
Verify Feeding Tube Placement: Confirm and document positioning per hospital protocol, such as chest radiograph or aspiration with pH testing. Reposition or remove if uncertain.
Recommended Maintenance
Visually inspect tube position before each administration using catheter markings.
Verify placement per facility protocol before use.
Flush with sterile water before and after administration.
Seal with tethered closure when not in use.
Recommended Feeding Tube Removal
Flush with 0.5cc–1cc sterile water.
Plug or clamp during withdrawal to help minimize aspiration risk.
Withdraw gently and discard according to institutional or regulatory guidelines.
Document removal.
Disposal: Follow local, state, and national regulations for disposal of potentially biohazardous components and accessories.
Specifications
| Brand | NeoMed |
| Manufacturer | Avanos Medical |
| Application | Nasogastric Feeding Tube with ENFit Connector |
| Material | PVC |
| Sterility | Sterile(Ethylene Oxide) |
| Primary Product Color | Orange/Purple |
| Tethered Cap | Yes |
| Radiopaque Stripe | Yes |
| ENFit Connector | Yes |
| Anti-IV connector | Yes |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Resources
Description
NeoMed PVC Feeding Tube With ENFit Connector is engineered to support gentle nasogastric and orogastric feeding by helping minimize tissue irritation commonly associated with oversized side ports or unfinished distal tips. The distal tip is designed to remain smooth, well formed, and open, while exposed surfaces are structured for accessibility and efficient cleaning. Attention to hub configuration and material flow pathways helps limit pooling within the distal end and recessed caps, factors that are often associated with bacterial transfer, making this feeding tube a dependable choice for neonatal and pediatric enteral nutrition in clinical environments.
Features
- BPA-free construction for added material safety.
- DEHP-free formulation to support sensitive patient populations.
- Centimeter graduations extending to at least 32 cm for accurate measurement.
- High-visibility insertion markings at each centimeter assist with placement confirmation.
- Tethered plug closure design enhances protection from ambient air exposure.
- Soft medical-grade polymers are selected to support developmental care and clinical performance.
- Wave-pattern closure plug promotes airflow through the hub, helping limit bacterial formation.
- Bottomless hub configuration reduces areas where HBM and formula could accumulate.
- Cost-effective design validated for indwell time up to thirty days to reduce repeat placements.
- Open distal tip without sharp edges or concealed cavities helps limit residual colonization.
- Three offset side ports are precision drilled to create smooth edges compared to larger cut holes used by other brands.
Intended Users: This device is designed for use by trained healthcare professionals.
Patient Target Group: This device is intended for patients of all age groups who require nasogastric or orogastric enteral feeding.
Indications for Use: This product is indicated for neonatal and pediatric patients to provide nutrition through nasal or oral gastric placement and is not intended for use beyond 30 days.
Contraindications: This device is designed to connect only with ENFit compatible connectors. It is not intended for use longer than 30 days or for patients with congenital GI tract anomalies above the stomach that require surgical intervention.
Device Description / Intended Purpose: The NEOMED Enteral Feeding Tube is intended for patients requiring intermittent or continuous enteral nutrition through nasogastric or orogastric pathways. Material options include PVC. Refer to product packaging for product-specific details.
Residual Risks and Side Effects: While outcomes vary depending on the clinical procedure, mitigations have been implemented to help minimize the following potential risks related to NEOMED usage.
Nasogastric Feeding Tubes and the associated NEOMED Extension Sets: abnormal reproductive development, allergic/systemic toxic reaction, over/under delivery of medication or nutrition, skin or tissue irritation involving the trachea, vocal cords, or gastrointestinal tract, infection, fever, sepsis, nausea, vomiting, bloating, diarrhea, delayed or prolonged therapy, pneumothorax, choking, aspiration, peritonitis, gastroenteritis, laceration, pain, and potential long-term carcinogenic or mutagenic effects.
MR Information
MR Safe
The NEOMED Enteral Feeding Tube contains only nonconducting, nonmetallic, and nonmagnetic components and presents no known hazards in MR imaging environments.
Clinical Benefits: Nasogastric and orogastric feeding tubes facilitate delivery of nutrition, fluids, and medications to the stomach through the nose or mouth.
Device Lifetime: Device performance has been validated for use up to 30 days.
Recommended Placement Procedure
Insertion Measurement: measure from the tip of the nose to the earlobe and then from the earlobe to midway between the xyphoid process and umbilicus. Centimeter markings on the catheter assist in placement.
A strip of sterile tape may be placed at the intended insertion depth for reference.
Insert the tube slowly into the mouth or nose, allowing the infant to swallow as advancement continues.
Advance to the predetermined length.
Precautions: Avoid unintended tracheal placement or insufficient insertion that leaves the proximal side hole outside the stomach. Secure the tube before checking placement.
Verify Feeding Tube Placement: Confirm and document positioning per hospital protocol, such as chest radiograph or aspiration with pH testing. Reposition or remove if uncertain.
Recommended Maintenance
Visually inspect tube position before each administration using catheter markings.
Verify placement per facility protocol before use.
Flush with sterile water before and after administration.
Seal with tethered closure when not in use.
Recommended Feeding Tube Removal
Flush with 0.5cc–1cc sterile water.
Plug or clamp during withdrawal to help minimize aspiration risk.
Withdraw gently and discard according to institutional or regulatory guidelines.
Document removal.
Disposal: Follow local, state, and national regulations for disposal of potentially biohazardous components and accessories.
Specifications
| Brand | NeoMed |
| Manufacturer | Avanos Medical |
| Application | Nasogastric Feeding Tube with ENFit Connector |
| Material | PVC |
| Sterility | Sterile(Ethylene Oxide) |
| Primary Product Color | Orange/Purple |
| Tethered Cap | Yes |
| Radiopaque Stripe | Yes |
| ENFit Connector | Yes |
| Anti-IV connector | Yes |
Warranty
- The product warranty is applicable as per the terms and conditions provided by the product manufacturer.
Please call us for specific details.
Return
- No returns will be accepted after 30 days from the date of shipment.
- All returns are subject to a restocking fee as per manufacturers terms and conditions.
- All returns must have an RGA number (Returned Goods Authorization), unauthorized returns will not be accepted.
- We do not guarantee fulfillment of any desired purpose or product suitability to the user and this will not be considered as a valid reason for return.
- The products must be new, unused condition, not tampered with, in original packaging and returned at the customers expense in the original packaging.
- If your return is not due to any manufacturing defect then the original shipping cost will be deducted from the total refund.
- Hygiene, bath and toilet items cannot be returned once opened or used.
- Standard manufacturer terms and conditions apply for return policy of this product.
Please call us for specific details.
Resources
Starts From
